|
PHNG Lab
PHNG Laboratory for Vascular Morphogenesis |
 |
|
Home
Research
Publications
Members
News
Jobs
Contact |
GENERAL RESEARCH INTERESTS Endothelial cell membrane dynamics during sprouting angiogenesis. |
Blood vessels have an important function in transporting oxygen and nutrients to support the metabolic demands of tissues during development, growth and physiology. Many blood vessels develop through the process of angiogenesis, when new vascular sprouts arise from pre-existing vessels. While many key molecules and signaling pathways have been identified to be essential for vessel formation, there is still a poor understanding of how pro- and anti-angiogenic signals are relayed to the cell’s machinery to drive changes in endothelial cell morphology and behavior that lead to the final pattern of the vasculature. |
| The Laboratory for Vascular Morphogenesis aims to unravel fundamental principles
that regulate endothelial cell behavior and dynamics during blood vessel
morphogenesis, using the zebrafish as a model system since is highly suited
for high resolution live imaging, advanced fluorescent microscopy techniques,
genetics, cell biology and chemical biology. We are particularly interested
in understanding how the cell cytoskeleton remodels the plasma membrane
to induce changes in endothelial cell shape. Our long-term goal is to understand
how upstream angiogenic signals and hemodynamic forces regulate endothelial
cell cytoskeleton to dictate cell behavior to shape and maintain blood
vessel architecture. |
| ENDOTHELIAL CELL PLASTICITY
|
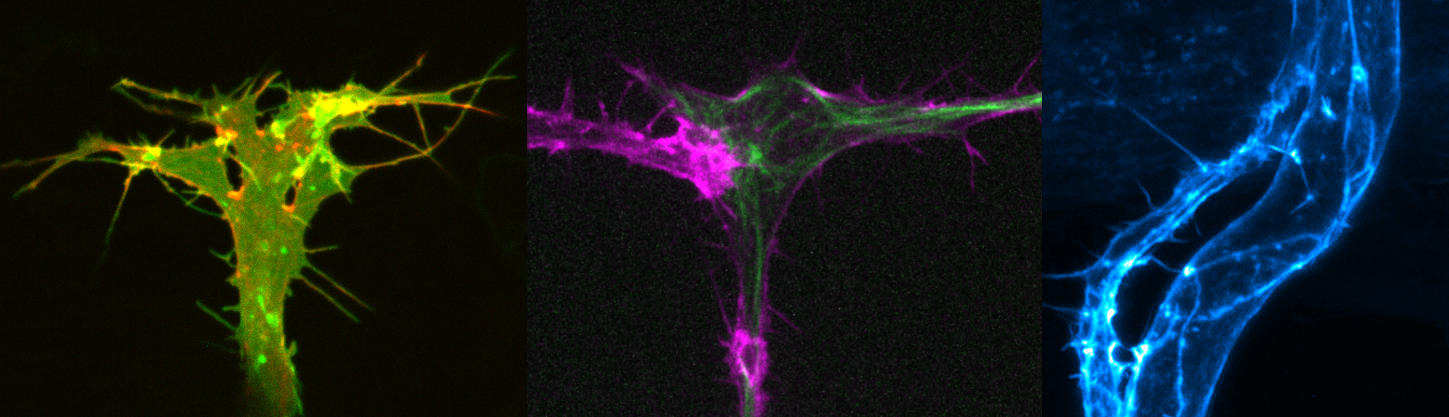
Dynamics of single endothelial cells during angiogenesis.
Endothelial cells generate diverse actin cytoskeletal
structures during vessel morphogenesis.
Filopodial, apical and junctional F-actin.Angiogenesis requires the tight coordination of many cellular processes. The expansion of a vascular network requires collective cell migration, proliferation, cell rearrangements and anastomosis. Concomitantly, the process of lumen formation takes place to render blood vessels functional. Thus, endothelial cells are highly plastic in their ability to change their morphology to drive specific cellular processes.
In order to understand how cell shape plasticity is achieved, we investigate how the actin cytoskeleton regulates endothelial membrane dynamics during angiogenesis. Previous studies showed that during the expansion of blood vessels, the generation of actin bundles in filopodia facilitates efficient collective cell migration and anastomosis (Phng et al., 2013). During lumen formation, transient polymerization of F-actin at the apical membranes controls lumen expansion (Gebala et al., 2016) while a pool of actin cables at endothelial cell-cell junctions stabilizes newly-formed tubules to produce a functional vascular network (Phng et al., 2015). Our work therefore demonstrates that actin cytoskeleton of different dynamics and localisation drive distinct steps of vessel morphogenesis.
| Future studies in the lab include: - Understanding the interplay between endothelial cell cortex, membrane dynamics and cell shape during migration and lumen formation. - Identifying molecular regulators of actin cytoskeleton that confer spatial and temporal specificity to the formation of distinct actin structures. - Understanding where and when tension is generated within the cell to drive cell shape changes. HAEMODYNAMIC FORCES AND ENDOTHELIAL CELL BEHAVIOR Once blood vessels become lumenized, endothelial cells are exposed to haemodynamic forces such as fluid shear stress and blood pressure. Recent work demonstrates that blood flow locally deforms the apical membrane of endothelial cells to generate inverse blebs during lumen formation (Gebala et al., 2016). In turn, endothelial cells counteract the deforming forces by triggering a repair mechanism. During the repair process, local and transient actomyosin activity is generated around the bleb cortex to retract the blebs, normalize apical membrane behaviour and allow controlled lumen expansion in the vessel. 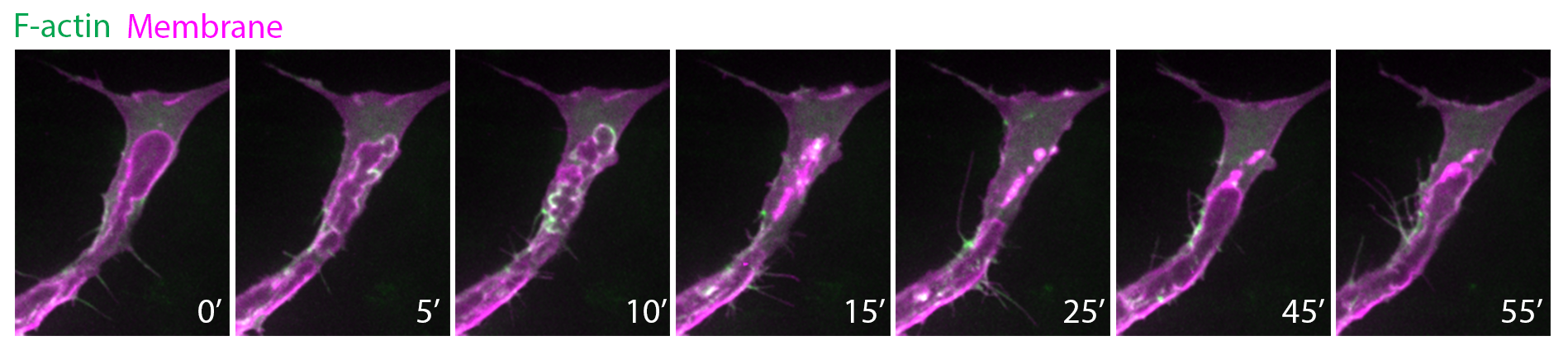 Actin polymerization (green) and myosin II activity at apical membranes (magenta) retract blebs, thereby controlling lumen expansion in zebrafish intersegmental vessels. To further understand the mechanobiology of endothelial cells, we are investigating: - How endothelial cell cortex is molecularly regulated to maintain cell shape and vessel architecture. - How endothelial cells sense and respond to changes in haemodynamic forces during development and homeostasis. Red blood cells (red) flowing in the dorsal aorta and posterior cardinal vein. Endothelial cells are in green. |
| LUMEN FORMATION In recent years, significant progress has been made in understanding the cellular mechanisms of lumen formation in angiogenic vessels. Identified mechanisms of lumen formation include cord hollowing, cell hollowing and transcellular lumen formation (Charpentier and Conlon, et al. 2014). However, the molecular regulation of lumen formation and maintenance are still largely elusive. We therefore seek to identify new molecular players by performing transcriptome profiling of endothelial cells and characterizing the role of potential candidates in vessel lumen formation. |
copyright©2018 Laboratory for Vascular Morphogenesis RIKEN Center
for Biosystems Dynamics Research